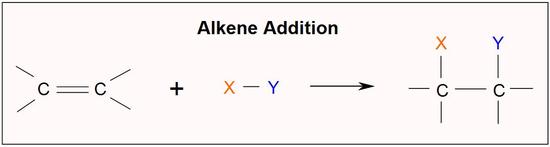
- A reaction in which two molecules combine to yield a single molecule or product is called an addition reaction or combination reaction.
- The addition reaction, in which alkenes (unsaturated hydrocarbon) add hydrogen in the presence of catalyst under high pressure and temperature to yield corresponding alkanes (saturated hydrocarbon), is known as hydrogenation.
- The catalysts commonly used are finely divided Platinum, Palladium or Nickel.
- Hydrogenation reaction carried in this manner is called catalytic hydrogenation.
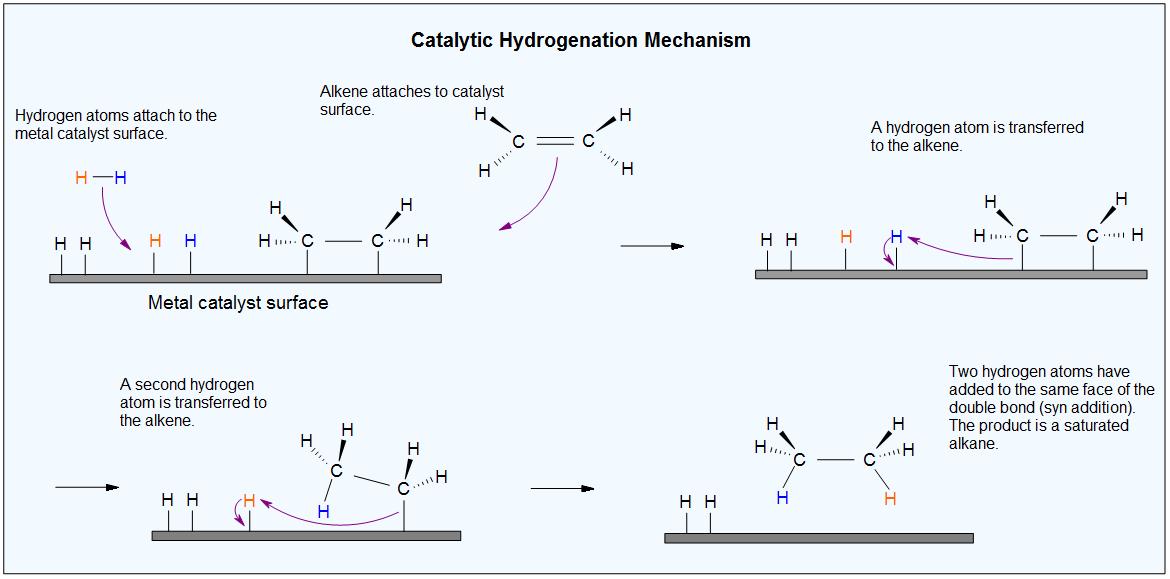
Mechanism of catalytic hydrogenation:
- The carbon-carbon double bond consists of a strong sigma (σ) bond and a weak pi (π) bond, therefore, the reaction, involves the breaking of this weaker π-bond.
- The loosely held π-electrons of the double bond serve as a source of electrons which are easily available to the reagents deficient in electrons (electrophiles or free radicals).
- With the presence of a metal catalyst like Pt, N etc, the H-H bond in H2 cleaves, and each Hydrogen atom attaches to the metal catalyst surface, forming metal-hydrogen bonds.
- The metal catalyst also absorbs the alkene onto its surface to which the H-atom is then transferred, forming a new C-H bond. A second hydrogen atom is transferred forming another C-H bond, resulting in the formation of a saturated alkane.
Applications of hydrogenation reaction:
- It is used in the production of edible fats (saturated fats) from liquid oils (unsaturated vegetable oils).
- Solid coal is converted to liquid form through the addition of hydrogen, that makes it available to be used as fuel.
- It is also used in food industry to increase the chemical stability of products like margarine which is a spread used for flavoring, baking, and cooking.