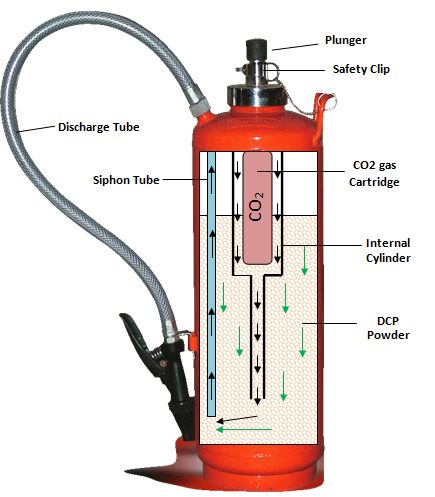
- A fire extinguisher is a protective device which is red in color and is used to extinguish fire.
- Red is the color associated with both danger and fire and hence metallic cases in fire extinguishers are colored red.
- Secondly,it is most easy to see red, especially in a darker environment, such as a smoke-filled room.
- Metallic vessel in a fire extinguisher contains sodium bicarbonate solution and a small bottle of concentrated sulfuric acid.
Working mechanism of a fire extinguisher:
- All fire extinguishers work on the basic principle of removing any of the three basic elements needed for combustion—oxygen, heat or fuel.
- Carbon dioxide is a gas which is neither combustible nor a supporter of combustion.
- When we need carbon dioxide gas to extinguish fire, the vessel is inverted.
- Once the vessel is inverted, the bottle of concentrated sulfuric acid strikes against the floor which makes the plug of the acid containing bottle fall down.
- Due to this, the acid comes in contact with sodium bicarbonate solution and react together giving carbon dioxide gas.
2NaHCO3 + H2SO4 (conc.) ———–> Na2SO4 + 2H2O + 2CO2
- The gas produced in the above reaction comes out through the nozzle with high pressure and puts off fire making a blanket like covering over the surface of the flame.
- The blanket like covering prevents the oxygen from coming in contact with the fire and extinguishes the fire.