
- The elements in which last electron enters the d- subshell of the penultimate (second outermost) energy level are called d-block elements.
- In other words, d- Block elements are the ones which in their elemental or compound forms have partially filled d- orbitals.
- They comprise the central region of the periodic table having elements from group 3 to 12 (III B to VIII (8/9/10) and IB and IIB.
- General valence shell electronic configuration is (n-1) d1-10 ns 1-2.
- These elements show the properties of variable valency, colored ions, complex formation, paramagnetic behavior, catalytic ability and pronounced metallic character.
Why d-Block elements are also called transition elements?
- d- Block elements are also called transition elements because they exhibit transitional behavior between highly reactive ionic compound forming s-block elements on one side, and mainly the covalent compound forming p-block elements on the other side.
- These elements have valence electrons both in their outermost and penultimate shells, because of which some of them show variable valency.
Classification of d- Block elements into four series:
- d- Block elements contain three complete rows called first, second and third transition series which involve filling of 3d, 4d, and 5d orbitals respectively and one incomplete row which is fourth row.
- First transition series (3d-series): It consists of elements from Scandium (21) to Zinc (30). These elements lie in the fourth period of the periodic table.
- Second transition series (4d-series): It consists of elements from Yttrium (39) to Cadmium (48). These elements lie in the fifth period of the periodic table.
- Third transition series (5-d series): It consists of elements Lanthanum (57) and from Hafnium (72) to Mercury (80). They lie in the sixth period.
- Fourth transition series (6-d series): It consists of elements Actinium (89) and from Rutherfordium (104) to Copernicium (112) and still counting. They lie in the seventh period which is still incomplete.
- 14 elements after Lanthanum, i. e. Cerium (58) to Lutetium (71), called Lanthanide series and 14 elements after Actinium, i. e. Thorium (90) to Lawrencium (103), called Actinide series are kept in inner transition elements below the periodic table. These inner transition elements are also called f-block elements.
Electronic configuration:
- General valence shell electronic configuration of all d- Block elements is (n-1) d1-10 ns 1-2.
- Actually, because of their incomplete inner d- orbitals, electronic configuration is considered to be (n-1) d1-9 ns 1-2, where (n-1) is the penultimate shell (second outermost shell).
- However, group IB (11) elements like Cu, Ag, Au etc. don’t correspond to the electronic configuration of transition elements because their inner d-orbitals are completely filled. Hence, these elements are regarded as the members of the typical transition elements.
- Some characteristic features of the electronic configuration of the transition elements are:
1. Atoms of all transition elements consist of an inner core of electrons having noble gas configuration.
21Sc= [Ar] 3d14s2 39Y= [Kr] 4d15s2 57La= [Xe] 5d16s2
2. The observed electronic configuration of the elements is different from their predicted configurations. The irregularities in the observed configurations of Cr, Cu, Mo, Pd, Ag and Au are explained on the basis of the concept of half-filled and completely-filled d-orbitals, which are relatively more stable than other d-orbitals. For e.g.
24Cr= [Ar] 3d44s2 and 29Cu= [Ar] 3d94s2 are the predicted electronic configurations of Chromium and Copper respectively, whereas,
24Cr= [Ar] 3d54s1 and 29Cu= [Ar] 3d104s1 are the observed electronic configurations, where Cr and Cu are relatively more stable.
General properties of transition elements:
- The electronic configurations of transition elements differ from one another only in the number of electrons in d- orbitals in the (n-1)th shell. The number of electrons in the outermost shell, ns, invariably 1 or 2.
- Hence, the properties of transition elements of any given period are not so much different from one another as those of the same period non-transition elements.
1. Metallic character:
- They have high binding energy as compared to s-block elements (alkali metals and alkaline earth metals).
- They are tough, malleable and ductile.
- They have high melting and boiling points and heat of vaporization is also high. Strong metallic bonding between unpaired electrons in the penultimate d-orbital of neighboring results in higher melting and boiling points.
- Group VIII (8/9/10) and group IB (11) are softer and more ductile as compared to the metals at the beginning of the transition series, which might be due to weaker metallic bondings.
- The metals have considerable mechanical strength, especially in alloys.
2. Atomic radii:
- The atomic radii of transition elements are smaller than those of the s- and p- block elements.
- Atomic radii of a particular transition series in a period, first decreases till the middle, become almost constant and then increases towards the end of the period.
- The decrease in atomic size in the beginning is attributed to the increasing nuclear charge.
- The balance of increasing nuclear charge by increased screening effect makes the atomic radii almost constant. Increase in atomic radii towards the end may be attributed to the electron-electron repulsions.
- The atomic radii increases going down the group, which is due to the introduction of an additional shell.
- Nearly equal radii of second and third transition series elements are due to a special effect called Lanthanide contraction.
*Lanthanide contraction: In the elements of Lanthanide series, the 5s- and 5p- orbitals penetrate the 4f- sub-shell, so the 4f- orbital is not shielded from the increasing nuclear charge, which causes the atomic radius to decrease. This decrease in atomic size throughout the series is called Lanthanide contraction.
3. Ionic radii:
- It follows the same trend as the atomic radii.
- It is seen that for ions of the given charge, the radius decreases slowly with the increase in atomic number. The gradual decrease in ionic radii across the series (period) is due to the increase in the effective nuclear charge.
4. Ionization potential (energy):
- It is the ease with which an electron can be removed from its isolated atom.
- The ionization energies of transition elements are higher than those of s-block elements but lower than p-block elements.
- In a particular transition series, ionization energy increases gradually on moving from left to right, which is due to the increase in nuclear energy. However, the increase in nuclear energy is partly cancelled by the increase in screening effect. Hence, the increase in ionization energy along the period is very small.
5. Oxidation state:
- Most of the transition elements exhibit variable oxidation states, i. e. they show variable valency in their compounds. The large number of oxidation states is related to their electronic configurations.
- Valence shell electronic configuration of transition elements is (n-1)d 1-10 ns 1-2.
- Since, the energy levels of (n-1)d and ns orbitals are quite close to each other, both the ns- and (n-1)d-electrons are available for bonding purposes. Hence, the number of oxidation states shown by these elements depends upon the number of d-electrons they have.
- For e.g. the outer electronic configuration of scandium (Sc) is 3d14s2. It exhibits an oxidation state of +2 in those compounds in which it uses both of its 4s-electrons. It can also exhibit oxidation state +3 when it uses two 4s-electrons as well as one d-electron in chemical bonding.
6. Standard electrode potentials and reducing properties:
- The standard electrode potential (oxidation) of a transition element is generally higher than that of standard hydrogen electrode (taken as zero). i. e. these elements (except Cu), generally react with acids to evolve hydrogen gas.
- They are good reducing agents. However, they are not as good reducing agents as the metals of group IA, IIA and IIIA.
- The poor reducing capacity of the transition metals is due to high heats of vaporization, high ionization potentials and low heats of hydration of their ions.
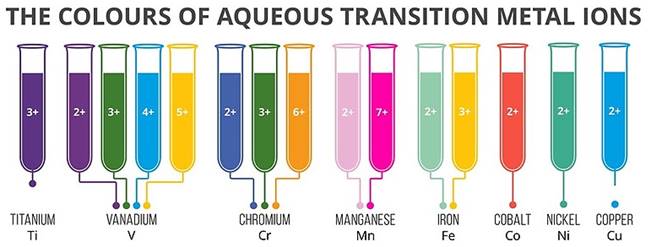
7. Color:
- Most of the compounds of transition metals are colored in the solid or in solution states. This is unlike the compounds of s- and p- block elements which are usually colorless or in any case not strongly colored.
- In the transition elements which have partially filled d-orbitals, the transition of electron can take place from one of the lower d-orbitals to some higher d-orbital within the same sub-shell. The energy required for this transition falls in the visible region.
- So, when visible light (wavelength of 3800-7600A0), falls on these complexes, they absorb a particular color from the radiation for the promotion of electron and the remaining colors are emitted. The color of the complex is due to this emitted radiation.
- Sometimes, if radiations of all the wavelengths (color) except one are absorbed, then the color of the substance will be the color of the transmitted radiation.
- Transition elements with completely filled d-orbitals generally don’t form colored compounds.
https://betrained.in/CBSE/12-Chemistry-Part-I/The-D-And-F-Block-Elements-Solution
8. Magnetic properties:
- Most of the transition elements and their compounds show paramagnetism. Paramagnetic substances are those which are attracted into a magnetic field.
- Paramagnetism arises by the presence of unpaired electrons in atoms, ions, complexes or molecules.
- The motion (spin motion or orbital motion) of unpaired electrons which are charged particles creates a magnetic field and hence provides magnetic property.
- This paramagnetic property first increases along the period in any transition series, becomes maximum around the middle and then decreases.
9. Catalytic properties:
- Most of the transition metals and their compounds have good catalytic properties. Platinum (Pt), iron (Fe), vanadium pentoxide (V2O5), nickel (Ni) etc. are important catalysts.
- Finely divided iron (Fe) acts as catalyst in the manufacture of ammonia by Haber’s process.
- Nickel powder is a good catalyst for hydrogenation of unsaturated organic compound such as oil. etc.
10. Complex formation:
- Transition metals are well known for complex compound formation.
- The tendency of transition elements to form complexes is due to two factors:
- These ions are very small in size and therefore, have high positive charge density which facilitates the acceptance of lone pairs of electrons from certain molecules (like CO, NO, NH3,H2O) etc. or with anions (like F–, Cl–, CN–), called ligands.
- They have vacant orbitals and these orbitals accept lone pairs of electrons donated by ligands to form coordinate covalent bonds.