Chemical reactions
Chemical reactions are the processes in which new substances with new properties are formed. During a chemical reaction, atoms of one element do not change into those of another element instead only a rearrangement of atoms takes place in a chemical reaction. For e.g. souring of milk, cooking of food, digestion of food in our body, ripening of fruits, rusting of iron etc.
- The substances which take part in a chemical reaction are called reactants.
- The new substances produced as a result of chemical reactions are called products.
Some of the important types of chemical reactions are:
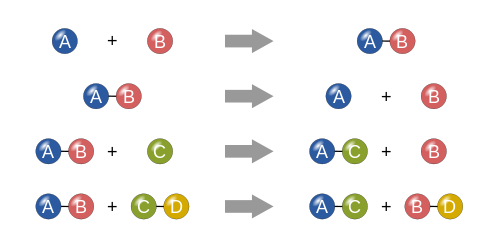
- Combination reactions or addition reactions:
Those reactions in which two or more substances combine to form a single substance, are called combination reactions.
A+B ————> AB
Examples:
- 2Na+ Cl2 ————-> 2NaCl
- Fe+ S ———-> FeS
- Decomposition reactions or dissociation reactions:
Those reactions in which a compound splits up into two or more simpler substances, are known as decomposition reactions.
AB ———> A+B
Examples:
- 2PbO2 ——–> 2PbO +O2 (in the presence of heat)
- 2KClO3 ———-> 2KCl + 3O2 (in the presence of heat and MnO2 )
- 2H2O ——–> 2H2 +O2 (in the presence of electricity)
- 2AgBr ——–> 2Ag + Br2 (in the presence of light)
- 2H2O2 ——–> 2H2O + O2 (in the presence of MnO2 as catalyst)
- Displacement reactions:
a) Single displacement reactions
Those reactions in which one element take the place of another element in compound, are known are displacement reactions. In general, a more reactive element displaces a less reactive element from its compound.
A+ BC ——-> AC + B
Examples:
- Zn+ H2SO4 ———> ZnSO4 + H2
- Fe+ CuSO4 ——–> FeSO4 + Cu
- KI +Cl2 ——–> 2KCl + I2
b) Double displacement reactions:
Those reactions in which two compounds react by an exchange of atoms or ions to from new compounds are called double displacement reactions.
AB +CD ——–> AD+ CB
Examples:
- CaCl2+2AgNO3 ———> 2AgCl + Ca(NO3)2
- NaCl (aq) + AgNO3 (aq) ———> AgCl + NaNO3
- HgCl2 (aq) + KI(aq) ——–> 2KCl + HgI2
- Acid- Base reactions or Neutralization reactions:
The reactions in which acids and bases of equal strengths react to neutralize each other properly toform neutral products like salt and water are known as neutralization reactions.
Acid + Base ——–> Salt + Water
Examples:
- HCl+ NaOH ——–> NaCl+ H2O
- H2SO4+ CaO ——–> CaSO4 + H2O
- Oxidation-Reduction reactions or Redox reactions:
The addition of oxygen or removal of hydrogen from a substance is called the oxidation of that substance whereas the process of reduction is just the opposite of oxidation, i.e.addition of hydrogen and removal of oxygen. Moreover, oxidation and reduction occur together hence also called redox reactions.
Examples:
- CuO + H2 ——–> Cu +H2O
- H2S +Cl2 ——–> S + HCl
- ZnO + C ——–> Zn + CO
- Exothermic reactions:
The reactions in which the heat is released are called exothermic reactions.
Examples:
- C+ O2 ——–> CO2 + heat
- 2H2+ O2 ——–> 2H2O + heat
- Endothermic reactions:
The reactions in which the heat is absorbed are called endothermic reactions. Generally, decomposition reactions are endothermic.
Examples:
- C+2S+heat ——–> CS2 (calcium disulphide)
- 2C + H2+ heat ——–> C2H2 (acetylene)
- 2NaNo3+ heat ——–> 2NaNO2+ O2
- NH4Cl +heat ——–> NH3 + HCl
- CaCO3+ heat ——–> CaO + CO2
Rate of chemical reactions:
The time taken by all of the reactants to be converted into the products completely is known as the rate of a chemical reaction.
Factors affecting the rate of the chemical reactions:
- Rate of a reaction normally increases with the increase in temperature.
- Rate of a reaction increases with the increase in concentration of the reactants.
- Rate of a reaction increases with the addition of a positive catalyst and decreases with the addition of a negative catalyst.
- Rate of a reaction increases with the increase in surface area of contact of the reactants
Catalyst: The chemical substance which affects or alters the rate of a chemical reaction but itself remains unchanged is called a catalyst. Catalysts are of two types:
1.Positive catalyst: The catalyst which increases the rate of a chemical reaction is called a positive catalyst.
2KClO3 ———> 2KCl + 3O2 (in the presence of heat and positive catalyst MnO2)
2.Negative catalyst: The catalyst which decreases the rate of a chemical reaction is called a negative catalyst.
2H2O2 ——–> 2H2O + O2 (in the presence of negative catalyst Glycerine)
* A promoter is a chemical substance which enhances the performance of a catalyst. e.g. Molybdenum (Mo)
N2 +3H2 ——–> 2NH3 (at 5000C and 200 atm with Fe as catalyst and Mo as promoter)
Types of chemical reactions