Classification of elements
Classification of Elements: Based on distinct physical properties like hardness, malleability & lustre a.Non-Metals b.Metals : Based on Chemical Properties: Alkali Metals: Metals which […]

Classification of Elements: Based on distinct physical properties like hardness, malleability & lustre a.Non-Metals b.Metals : Based on Chemical Properties: Alkali Metals: Metals which […]

Valency: Combining capacity of an element or a radical with another element or radical to form a compound or molecule Total number of electrons lost, […]

Chemistry: Branch of Science which deals with the study of matter, its composition and properties Matter: anything that occupies space and has mass e.g. stone, […]

S.N. Metals Non-metals 1. Metals are usually solids at room temperature. {Exception: Mercury (Hg) and Gallium (Ga) are liquid metals}. Non-metals usually are solids or […]
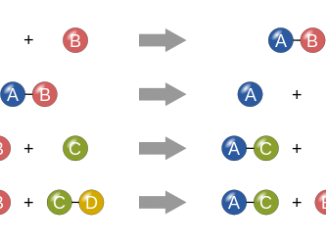
Chemical reactions Chemical reactions are the processes in which new substances with new properties are formed. During a chemical reaction, atoms of one element do […]

Silver: Symbol :- Ag Atomic number :- 47 Atomic weight :- 107.88 amu Valency :- 1 Position in the periodic table :- group IA (d-block […]

Metals form an important class of elements. They are widely used in our daily life for a large number of purposes. They are the elements […]

One hundred and fifteen different elements have been discovered so far, which combine to form a huge number of compounds. On the basis of their […]

Modern periodic table was proposed by Henry Moseley in 1963. He found that the atomic number is the more fundamental property which led to the […]
Copyright © 2026 | WordPress Theme by MH Themes