
What is Triacylglycerol (TAG)?
- Fatty acids and glycerol are the end products of digestion of lipids in our body.
- Fatty acids are organic acids whereas glycerol is a tri-hydroxy alcohol.
- The ester compound formed after the reaction between fatty acids and glycerol is called acyl glycerol.
- Triacylglycerols or triglycerides are formed by ester linkage of fatty acids to three alcohol groups in glycerol. Animals can synthesize and store large quantities of Triacylglycerols in the form of fats in adipose tissue, to be used later as fuel. TAG is also the main constituent of vegetable oils.
- TAGs are hydrophobic and hence are not soluble in the aqueous environment of the bloodstream. They are carried by macromolecular particles called lipoproteins for their transportation in blood.
Biosynthesis of TAG:
- Most of the fatty acids either synthesized in body or ingested through diet by an organism have one of two fates;
- incorporation into triacylglycerols for the storage of metabolic energy
- incorporation into the phospholipid components of membranes
- The organism’s current needs determine the alternative fates of fatty acids.
- During rapid growth, synthesis of new membranes requires the production of phospholipids, whereas, when an organism has a plentiful food supply but not actively growing, it shunts most of its fatty acids into storage fats also called adipose tissue.
- Both of these pathways begin at the same point, i.e. the formation of fatty acyl esters of glycerol. Hence, triacylglycerols and glycerophopholipids are synthesized from the same precursors.
- The major sites for the synthesis of TAG are liver, adipose tissues, intestinal mucosa, mammary glands and muscles.
- In animal tissues, TAGs and glycerophopholipids such as phosphatidylethanolamine share two precursors; fatty acyl-CoA and L-glycerol 3-phosphate and several biosynthetic steps.
- Formation of L-glycerol 3-phosphate:
- The vast majority of the glycerol 3-phosphate is derived from the glycolytic intermediate dihydroxyacetone phosphate (DHAP) by the action of cytosolic NAD-linked glycerol 3-phosphate dehydrogenase. In liver and kidney, a small amount of glycerol 3-phosphate is also formed from glycerol by the action of glycerol kinase.
- Formation of fatty acyl-CoAs:
- The other precursors of TAGs are fatty acyl-CoAs, formed from fatty acids by acyl-CoA synthetases, the same enzymes responsible for the activation of fatty acids for β-oxidation.
Steps in biosynthesis of TAG:
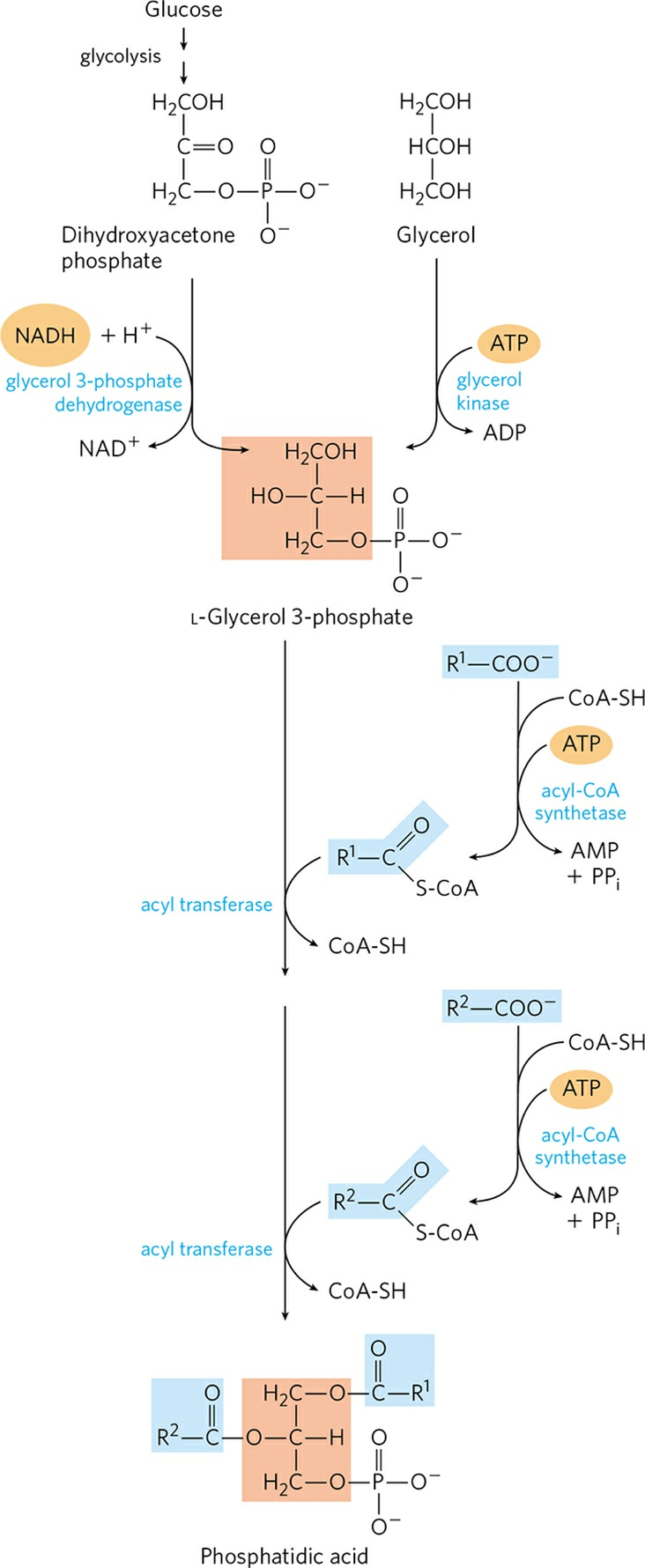
- Conversion of L-glycerol 3-phosphate to diacylglycerol 3- phosphate (Phosphatidic acid):
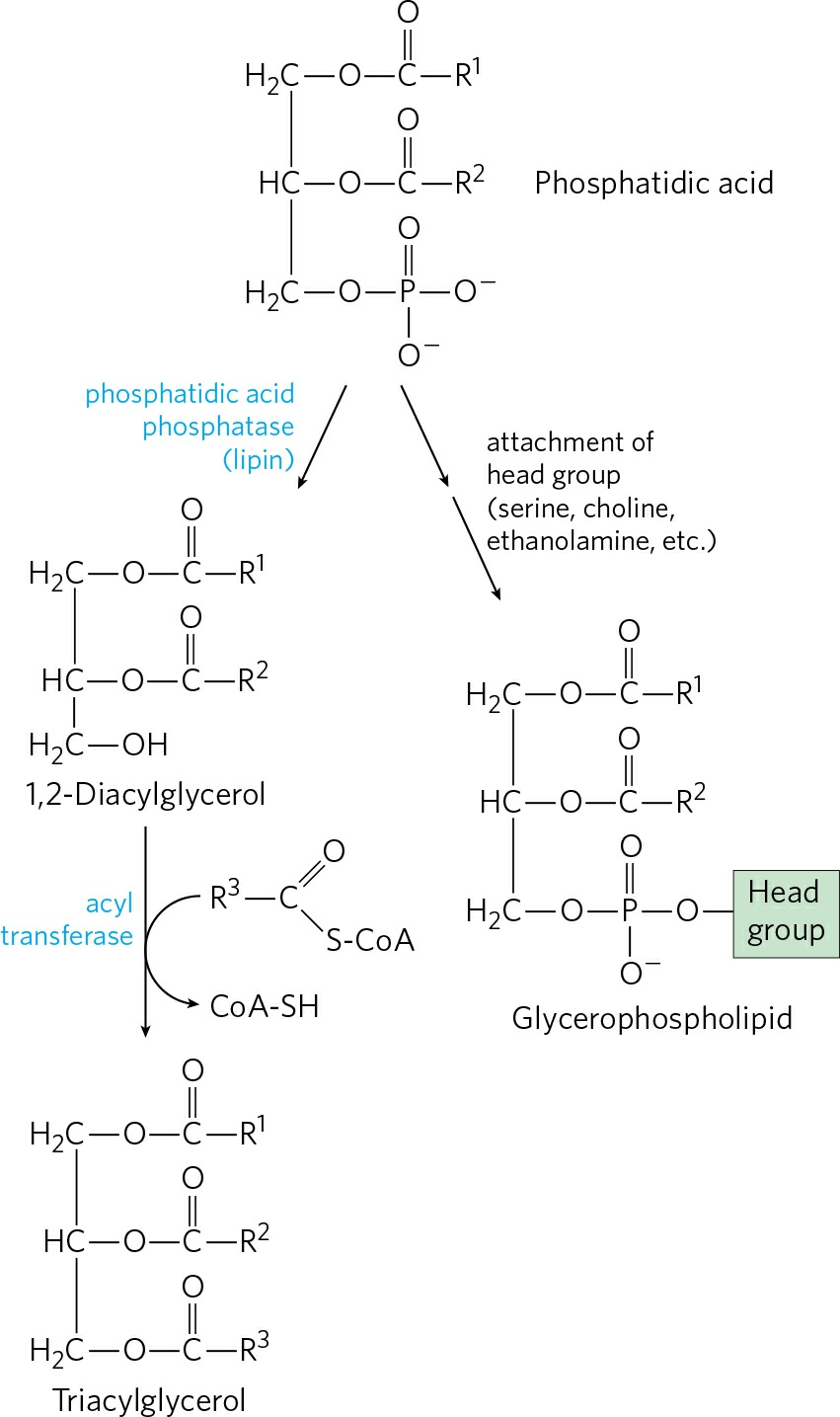
- The first step in the biosynthesis of TAGs is the acylation of the two free hydroxyl groups of L-glycerol 3-phosphate by two molecules of fatty acyl-CoA to yield diacylglycerol 3- phosphate, which is more commonly known as phosphatidic acid or phosphatidate. Phosphatidic acid is found in minute amount in cells but is a central intermediate in lipid biosynthesis. It can be converted either to a TAG or to a glycerophopholipid.
- Hydrolysis of Phosphatidic acid into 1,2-diacylglycerol:
- In the second step of TAG biosynthesis, phosphatidic acid thus formed is hydrolyzed by phosphatidic acid phosphatase to form a 1,2-diacylglycerol.
- Conversion of diacylglycerols into triacylglycerols:
- Diacylglycerols obtained from step 2 are then converted to Triacylglycerols with the incorporation of a third fatty acyl-CoA by an enzyme acyl transferase, the process called transesterification.
Regulation of TAG biosynthesis in animals:
- The synthesis and breakdown of TAG are regulated by several hormones
- Insulin stimulates the conversion of dietary carbohydrates and proteins to fat. Individuals with diabetes mellitus lack insulin; in uncontrolled disease, this results in diminished fatty acid synthesis, and the acetyl-CoA arising from catabolism of carbohydrates and proteins is shunted to ketone body production.
- In mammals, TAG molecules are broken down and resynthesized in a TAG cycle during starvation. Some of the fatty acids released by lipolysis of TAG in adipose tissue pass into bloodstream, and the remainder are used for the resynthesis of TAG. Some of the fatty acids released into the blood are used for energy (e.g. in muscle), and some are taken up by liver and used in TAG synthesis. The TAG formed in liver is transported in the blood back to adipose tissue, where fatty acid is released by extracellular lipoprotein lipase, taken up by adipocytes, and reesterified into TAG.
Fates of TAG in the Liver and Adipose Tissue:
- In the white adipose tissue, TAG is stored in a nearly anhydrous form as fat droplets in cytosol of the cell. It is also the body’s major source of energy
- Stores are released following binding of hormones, especially epinephrine, transduction through the Cyclic adenosine monophosphate (cAMP) pathway, and activation of hormone-sensitive lipase.
- Little TAG is stored in liver while most is packed with other lipids and Apo protein to form lipoprotein particles called VLDL which are released into blood.
Functions of TAG:
- Triglycerides provide our body with energy, but their main function is to store energy for later use.
- Plants store energy in fats and oils. Oils are particularly common in seeds, where the stored energy helps seedlings during germination, until they can exploit solar energy through photosynthesis.
- Fat serves as a protective cushion and provides structural support to help prevent injury to vital organs such as the heart, liver, kidneys, and spleen.