
- Antigen processing and presentation are the means by which protein antigens become associated with self MHC molecules for presentation to T-cells with appropriate receptors. It can be broken down into two components;
- Antigen processing: It converts large antigenic proteins into short peptides that can be displayed on the cell membrane together with an MHC molecule (either class I or class II) and recognized by T-cells.
- Antigen presentation: In this process, certain cells in the body, especially antigen presenting cells (APCs) express processed antigen on their cell surface along with MHC molecules that are recognized by T-cells.
- CD8+ TC cells recognize antigens presented along with class I MHC molecule, whereas, antigens presented along with class II MHC molecule are recognized by CD4+ TH
- There are 2 different pathways used by our immune system to eliminate intracellular and extracellular peptide antigens:
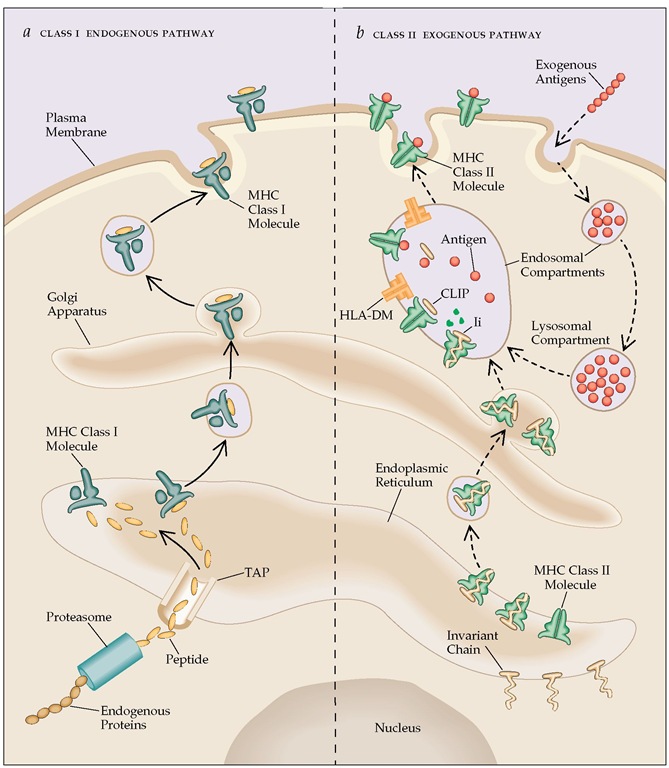
A. Endogenous Pathway or Cytosolic pathway:
- Intracellular or endogenous antigens, those generated within the cell, e.g. viral protein synthesized in an infected cell, tumor cells and intracellular pathogens ( tuberculosis, Histoplasma capsulatum) are processed through this pathway.
- Thus processed antigens are presented on the cell membrane with class I MHC molecules to CD8+ Tc cells for degradation.
Steps involved in Cytosolic pathway:
1. Proteolytic degradation of large antigenic protein into peptides:
- MHC molecules cannot bind large sized intracellular protein antigens. Therefore, they have to be degraded into short peptides of about 8-10 amino acids.
- Cytosolic proteolytic system or peptidase complex called proteasomes which are present in all the cells help in degradation of such proteins.
- The large (20S) proteasome is a barrel shaped complex composed of 14 sub-units symmetrical rings. However, not all of these sub-units have protease activity.
- Large proteins enter the proteasome through narrow channels present at its two ends.
- A small protein called ubiquitin is attached to many proteins targeted for proteolysis.
- This ubiquitin-protein complex is then degraded by a 26S proteasome, a multifunctional protease complex (20S proteasome + 19S regulatory component).
- The central hollow of the proteasome is the site where the degradation of ubiquitin protein complex is thought to occur.
2. Translocation of peptides from cytosol to rough Endoplasmic Reticulum (RER):
- TAP (transporter associated with antigen processing) proteins transport small peptides (8-10 amino acids) formed in the cytosol by proteasome complex to rough endoplasmic reticulum (RER).
- This process requires the hydrolysis of ATP.
- TAP is a heterodimer of TAP1 and TAP2, which is anchored in the membrane of RER and has affinity for peptides having 8-16 amino acids.
- Class I MHC can most effectively bind peptides of nine amino acids that are formed by trimming the peptides with the help of endoplasmic reticulum amino-peptidase (ERAP).
- TAP also favors peptides having amino acids with hydrophobic or basic carboxyl terminal, also preferred by class I MHC molecules.
3. Assembly of peptides with class I MHC molecules:
- Class I MHC molecule has two components, the α-chain and β2 microglobulin which are synthesized on polysome along the rough endoplasmic reticulum (RER).
- Class I MHC molecule has a stable assembly of α-chain and β2 microglobulin and exit the RER only when its binding groove consists of a peptide called molecular chaperones.
- Molecular chaperones facilitate the folding of polypeptides.
- The first molecular chaperone involved in the assembly of class I MHC is calnexin which is a resident membrane protein of RER.
- It associates with free class I α-chain and promotes its folding.
- The binding of β2-microglobulin with α-chain, releases calnexin from class I MHC. Class I MHC then associates with other chaperones calreticulin and tapasin (TAP-associated protein).
- TAP transporter, carrying peptides is brought to the close proximity of class I MHC molecule by tapasin after which Class I MHC acquires the antigenic peptide.
- An additional protein with enzymatic activity, ERp57, allows the release of class I MHC after acquiring the antigenic peptide. This is possible because ERp57 form disulfide bond with tapasin and non-covalently associates with calreticulin to stabilize the interaction.
- As a consequence, peptide bound class I MHC is released from the complex of calreticulin, tapasin and ERp57, which then exits from RER and displays on the cell surface via golgi complex.
http://what-when-how.com/acp-medicine/cell-cell-interactions-cytokines-and-chemokines-in-immune-response-mechanisms-part-1/
B. Exogenous or Endocytic pathway:
- Extracellular antigens like bacteria (those taken up by endocytosis) are processed in the endocytic pathway.
- At first, antigen presenting cells (APCs) internalize the extracellular antigen by phagocytosis or endocytosis or both.
- Dendritic cells and macrophages internalize antigens by both endocytosis and phagocytosis, whereas APCs which are non-phagocytic or poorly phagocytic, internalize the antigen by receptor mediated endocytosis or pinocytosis. e. g. B cells.
- The processed antigens are presented on the cell membrane with class II MHC molecules which are recognized by CD4+ TH cells.
Steps involved in endocytic pathway:
1. Peptide generation from internalized antigen inside endocytic vesicles:
- Endocytic pathway involves the degradation of internalized antigens into peptides within acidic compartments.
- Three increasingly acidic compartments; early endosomes (pH 6-6.5), late endosomes or endo-lysosome (pH 5-6) and lysosomes (pH 4.5-5) are involved in this pathway.
- The internalized antigen gradually moves from early to late endosomes and finally to lysosomes, where it encounters with hydrolytic enzymes like hyrolases, proteases, nucleases, lipases etc. and the environment is increasingly acidic.
- Oligopeptides with 13-18 amino acids are generated from internalized antigen which then binds with class II MHC. This binding protects the oligopeptide from further proteolysis.
- The mechanism behind the movement of internalized antigen from less acidic to more acidic compartment has not been well explained.
- It has been suggested that:
- Early endosome move from periphery to inward to become late endosome and finally lysosomes.
- Small transport vesicles may carry antigen from one compartment to the next.
2. Transport of class II MHC molecule to endocytic vesicles:
- Since antigen-presenting cells express both class I and class II MHC molecules, something must prevent class II MHC molecules from binding to the same set of antigenic peptides as the class I molecules.
- A trimeric protein called invariant chain (Li, CD74) interacts with the peptide-binding cleft of class II MHC, preventing any endogenously derived antigen from binding to the cleft.
- Invariant chain, in its pre-assembled form is associated with three pairs of class-II α and β-chains when class II MHC molecules are synthesized within RER.
- The invariant chain also helps in the folding of the class II αand β-chains, their exit from the RER and the subsequent routing of class II MHC molecule to endocytic compartments from the trans-golgi network.
- The invariant chain contains sorting signals in its cytoplasmic tail that direct the transport of the class II MHC complex from the trans-golgi network to the endocytic compartments.
3. Assembly of peptides with Class II MHC molecules:
- Class II MHC-invariant chain complexes are transported from RER through trans-golgi network to endocytic compartments; moving from early endosome to late endosome and finally to lysosome.
- The proteolytic activities inside each acidic compartment gradually degrade the invariant chain leaving a short fragment of invariant chain called CLIP (Class-II associated invariant chain).
- CLIP physically occupies the peptide-binding cleft of class II MHC molecule and prevents any premature binding of antigenic peptides.
- HLA-DM (a non-classical class II MHC), is required to catalyze the exchange of CLIP with antigenic peptides.
- HLA-DM is a heterodimer of α and β-chains, which unlike other class II MHC is not expressed at the cell membrane.
- The reaction between HLA-DM and the class II-CLIP complex facilitating exchange of CLIP for another peptide is impaired in the presence of HLA-DO, which binds to HLA-DM and lessens the efficiency of the exchange reaction.
- Lower pH in endocytic compartments weakens the association of HLA-DM and HLA-DO and hence increases the possibility of antigenic peptide binding despite the presence of HLA-DO.
- Like in class I MHC, peptide binding maintains the structure and stability of class II MHC molecules.
- This peptide-class II MHC complex is transported to the plasma membrane where neutral pH enables the complex to assume the compact and stable form with strongly bound peptide difficult to replace with another peptide at physiological conditions.