
- Antibodies are globulin proteins present in blood that boost our immune system. Hence, these proteins are also called immunoglobulins.
- Antibodies react specifically with antigens and help in their deactivation and elimination from our body.
- Antigens are any foreign particles to our body, usually proteins or polysaccharides that stimulate the immune system to produce antibodies.
- B lymphocyte produces antibodies when an antigen interacts with it through immunoglobulin surface receptors.
Basic structure of antibody:
- Although there is some remarkable variability in the antigen binding regions, all antibodies share the same basic structural properties.
https://www.sinobiological.com/resource/antibody-technical/antibody-structure-function
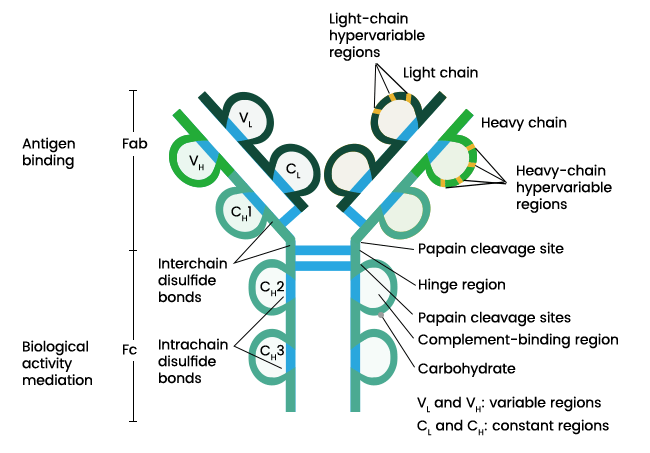
- Heavy-chains and light chains:
- The simplest antibody molecule is Y-shaped and consists of four polypeptide chains: two heavy (H) chains and two light (L) chains.
- L chains have a molecular weight of approximately 25 KDa whereas H chains have a molecular weight of approximately 50 KDa.
- L and H chain are covalently linked to each other by disulfide bonds. Similarly, disulfide bondsformed between cysteine residues in the carboxy terminus of the light chains link them together and disulfide bonds formed between CH1 domain of the heavy chains covalently link them together.
- Each chain is divided into regions or domains consisting of around 110 amino acid residues. The light chain has two domains (VL and CL) and the heavy has four (CH1, CH2, CH3 and VH).
- Both heavy chains and light chains consist of two terminals; amino-terminal and carboxy-terminal.
- The N-terminal domains on both the heavy and light chains are known to be variable in amino acid sequence composition and hence called variable domains (VL and VH) whereas, the other domains in both the chains are called constant for a similar reason (CL, CH1, CH2, CH3).
- The two major light chain constant region sequences are referred to as K (kappa) or λ (lambda).
- Sequences of heavy chain constant regions are of 5 types named with Greek letters; μ(mu), 𝛿 (delta), γ (gamma), ε (epsilon), and α (alpha) which divide immunoglobuins into 5 classes IgM, IgD, IgG, IgE and IgA repectively.
- Hinge region:
- Antibody molecules are flexible, which permits them to bind to different array of antigens.
- Thehinge region located between CH1 and CH2 in certain isotypes provides this flexibility to the antibody molecule.
- If an antibody molecule is treated with a protelytic enzyme, papain, peptide bonds in the hinge region are broken.
- Hyper-variable regions and complementarity determining regions:
- Within the variable regions of both L and H chains are sub-regions consisting of extremely variable amino acid sequences called hyper-variable regions that form the antigen-binding site.
- These hyper-variable regions form the area of the antibody molecule complementary in structure to the antigenic determinant of epitope and are therefore also known as complementarity determining regions (CDRs).
- Fab and Fc regions:
- The antigen-binding site of the antibody molecule is called Fab region. The arms of “Y” in antibody are the antigen binding sites.
- The region of antibody where antigen doesn’t bind and crystallizes easily is called Fc region (fragment crystallizable).
- Fc region of the antigen-coupled antibody binds to Fc receptors on phagocytic or cytolytic cells, or to immune effector molecules to carry out the biologic functions. Fc region also helps in complement activation.
You may also want to see: Five major classes of Antibodies and their Biological Activities
Biological function of Antibodies:
- Antibodies have three main functions:
- Neutralization of the antigen: The binding of antibodies with antigens (pathogens and toxins) will inactivate them or neutralize their effect.
- Activation of complement system: Antibody-antigen complex activates the proteins in complement system and help in the lysis of bacterial cells by punching holes in the cell wall. i. e formation of membrane attack complex (MAC).
- Opsonization: Antibodies help in chemical modification of antigens that can be easily recognized by the receptors on the surface of phagocytic cells followed by phagocytosis.