
Silver:
- Symbol :- Ag
- Atomic number :- 47
- Atomic weight :- 107.88 amu
- Valency :- 1
- Position in the periodic table :- group IA (d-block element)
Ores:
- Argentite (silver glance) :- Ag2S
- Horn silver :- AgCl
- Silver copper glance :- Ag2S, Cu2S or (AgCu)2S2
Physical properties:
- It is shining white metal.
- It is highly malleable and ductile.
- It is a good conductor of heat and electricity.
- Its melting point is 9600C and boiling point is 19550
- Its density is 10.5 gm/cm3.

Uses:
- It is used for table-ware, artistic objects and coinage.
- It is used in electroplating.
- In the photographic industry, silver bromide (AgBr) is used in the production of photographic plates, films and printing paper.
- It is used in silvering mirrors.
- Silver nitrate (AgNO3) is soluble in water and is used in silver plating.
- Silver oxides are used to make small, powerful batteries for calculators, hearing aids and watches.
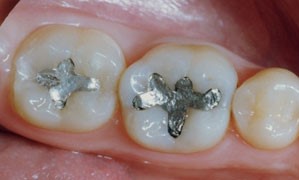
- Dentists fill cavities in teeth with silver amalgam, a mixture of silver, tin and mercury.
Gold:
- Symbol :- Au
- Atomic number :- 79
- Atomic weight :- 197.2 amu
- Valency :-1 or 3
- Position in the periodic tabl :- group IB (d-block element)
Ores:
- Gold occurs native in alluvial deposits as fine particles mixed with sand.
- It is found in certain river beds or embedded in quartz veins.
- It is usually accompanied by impurities of silicon, copper, mercury, zinc and lead.

Physical properties:
- It is yellow colored with soft metallic glow.
- It is ductile and malleable.
- It is a good conductor of heat and electricity.
- It melts at 10690C and boils at 25300
- Its density is 19.3 gm/cm3.
- Is it not brittle like other metals and doesn’t rust or tarnish.
Uses:
- Gold is used as a form of international money. All countries accept gold in payment for international debts.
- It is too soft for making tools, but is used for making coins and medals.
- It is used for making jewelry and for other decorative objects and purposes.
- It is used to fill cavities in teeth. These alloys are used by dentists to make bridges and crowns.
- It is used in making gold leaf electroscope.
- It is used for gold plating over other metals.
- Is is used in making compounds of gold which are used in photography.

Reactivity of gold:
- Gold is an unreactive metal and hence it is not attacked by atmospheric oxygen, water or steam and the common mineral acids.
- It lies below the hydrogen in the electrochemical series and hence doesn’t displace hydrogen from dilute mineral acids (HCl, HNO3 and H2SO4).
- Gold dissolves in aqua regia to form auric chloride. Aqua regia is a mixture of 3 parts conc. HCl and one part conc. HNO3.