Principle:
This test is performed to determine or identify the presence of an enzyme cytochrome oxidase (of the electron transport chain) in bacterial cells. The substrate used is tetramethyl-p-phenylenediamine dihydrochloride, which is oxidized to a purple colored end product called indophenol by the enzyme oxidase. The development of a dark purple color is a positive test that indicates the presence of oxidase, whereas if the enzyme is not present, the reagent remains reduced and is colorless.
Procedure:
- Take a filter paper and moisten it with the substrate i.e. 1% tetramethyl-p-phenylenediamine dihydrochloride or select a commercially available paper disk that has been impregnated with the same substrate.
- Remove a small portion of a bacterial colony (preferably not more than 24 hours old) from the agar surface with a sterile platinum wire or wooden stick.
- Rub the sample on the filter paper or commercial disks.
- Observe the inoculated area of the paper of disks for the color change to deep blue or purple within 10 seconds because timing is very critical.
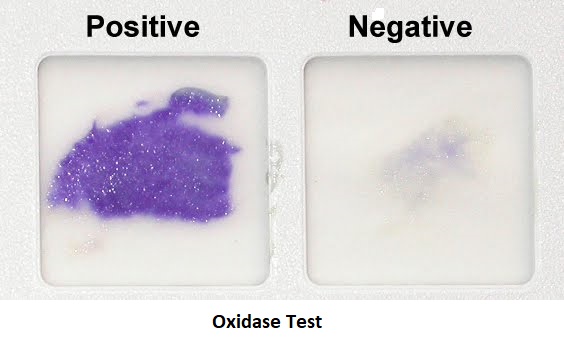
Result interpretation:
- Positive result: Development of a dark purple color within 10 seconds of inoculation. E.g. Neisseria gonorrhoeae, Vibrio cholera, Pseudomonas are oxidase positive.
- Negative result: No change in color (i.e. no blue color seen) e.g. Members of family Enterobacteriaceae like E.coli are oxidase negative.
Precautions:
- Avoid using Nickel-base alloy wires containing chromium and iron (nichrome) to rub the colony paste onto the filter paper as this may give false positive results
- Interpret the results within 10 seconds.
- Lactose in MacConkey Agar and Sucrose in TCBS are fermentable carbohydrates that results in acidification of medium giving false negative oxidase. Hence, oxidase test must be performed from 5% Sheep blood agar or another medium without a fermentable sugar.
Oxidase test by Kovac’s method (Principle, Procedure, Result Interpretation and Precautions)