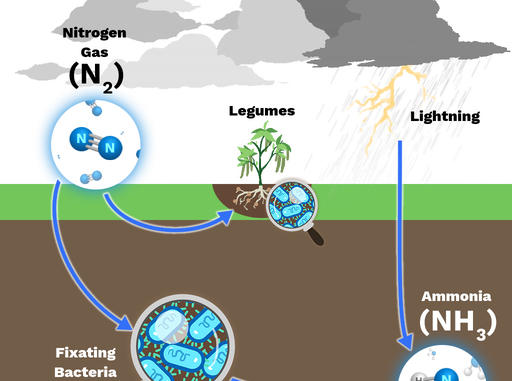
What is nitrogen fixation?
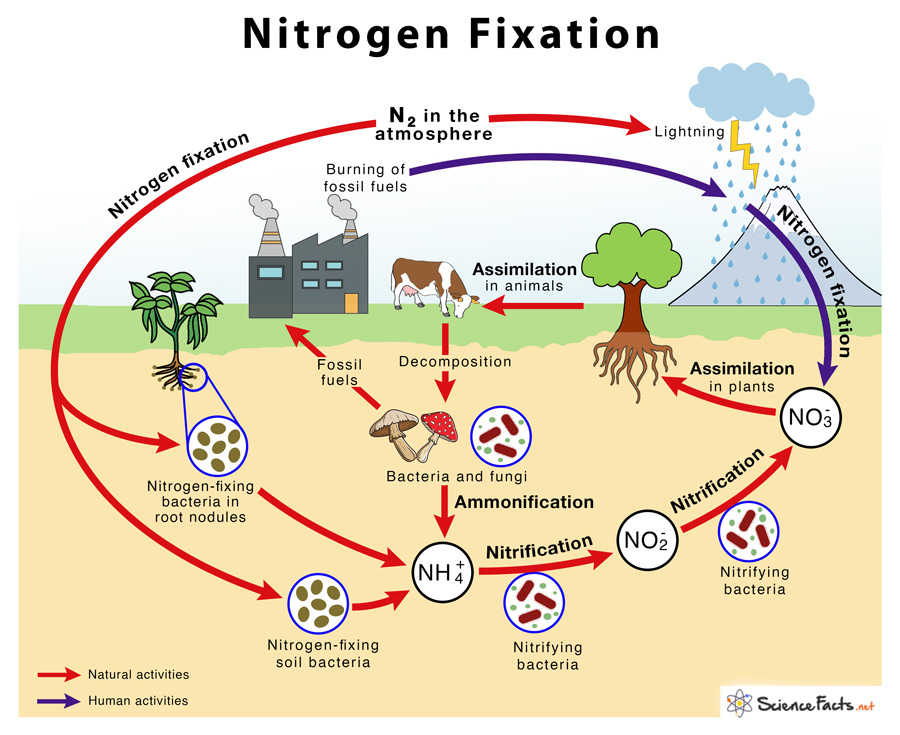
- The phenomenon of conversion of free nitrogen into nitrogenous salts to make it available for absorption by plants is called nitrogen fixation.
- On the basis of agents through which the nitrogen is fixed, the fixation of nitrogen is divided into two types:
- Physical nitrogen fixation
- Biological nitrogen fixation
Physical nitrogen fixation:
- Out of total nitrogen fixed by natural agencies, approximately 10% of this occurs due to physical processes such as lightning (i.e. electric discharge), thunder storms and atmospheric pollution.
- The lightning and UV-radiations in the atmosphere favors the combination of gaseous nitrogen and oxygen to form nitric oxide (NO). The nitric oxides are again oxidized with oxygen to form nitrogen peroxide (NO2).
N2+O2 ————> 2NO (During lightning and thunderstorm)
2NO+O2 ————–> 2NO2 (Oxidation)
- This nitrogen peroxide can combine with water during rains to form nitrous acid and nitric acid. The acids fall on the ground along with rain water and react with alkaline radicals to produce water soluble nitrates (NO3–) and nitrites (NO2–).
2NO2 + H2O ——> HNO2 + HNO3
HNO3 + Ca or K salts —————-> Ca or K nitrates
- The nitrates are soluble in water and are directly absorbed by the plants.
Biological nitrogen fixation:
- The conversion of atmospheric nitrogen (molecular nitrogen) into inorganic or organic usable forms with the help of living organisms is called biological nitrogen fixation.
- This process is carried by two main types of microorganisms;
- those which are free living or asymbiotic
- and those which live in close symbiotic association with other plants
- However, a third category of microbes which fix nitrogen in association with roots of cereals and grasses has been recognized. It is called associative symbiotic nitrogen fixation.

Asymbiotic nitrogen fixation:
- A large number of free living bacteria and cyanobacteria fix atmospheric nitrogen into usable form.
- These microorganisms can be categorized as follows:
- Free living aerobic nitrogen fixing bacteria. e.g. Azotobacter, Beijerinckia, Derxia etc.
- Free living anaerobic nitrogen fixing bacteria. e.g. Clostridium pasteurianum, Bacillus etc.
- Free living photo-autotrophic nitrogen fixing bacteria. e.g. Chlorobium, Rhodopseudomonas, Rhodospirellum etc.
- Free living chemosynthetic nitrogen fixing bacteria. e.g. Desulfovibrio
- Free living nitrogen fixing Cyanobacteria: A large number of heterocystous Cyanobacteria or blue green algae are known to fix atmospheric nitrogen. e.g. Nostoc, Anabaena, Cylindrospermum, Trichodesmium, Calothrix etc. Some of the free living Cyanobacteria fix nitrogen in rice fields (e.g. Aulosira fertilissima), some fix nitrogen actively in sugarcane and maize fields (e.g. Cylindrospermum)
Symbiotic biological nitrogen fixation:
- Many bacteria and Cyanobacteria fix atmospheric nitrogen in symbiotic association with other plants. Some common examples are:
- Rhizobium leguminosarum (Bacteria) in association with roots of leguminous plants.
- Nostoc (cyanobacteria ) in association with a bryophyte (Anthoceros).
- Anabaena azollae (Cyanobacteria) in association with a water fern (Azolla).
- Anabaena and Nostoc in association with coralloid roots of a gymnosperm, Cycas.
Symbiotic nitrogen fixation in leguminous plants:
- One of the most important nitrogen fixing bacteria, Rhizobium fixes nitrogen in symbiotic association with roots of leguminous plants like pea, beans, clover, Alfa-alfa, lupines etc.
1. Chemical interaction and entry:
- Initially the bacteria grow in the soil near the roots of higher plants where they fail to fix nitrogen. When these bacteria come in contact with the roots of leguminous plants, they interact chemically and enter into the roots through root hairs.
- Entry of bacteria into the root hair depends on various chemical signals sent from the root hair.
2. Formation of infection thread:
- The enzymes produced by bacteria degrade the parts of the root hair cell wall which produces a thread like structure called infection thread.
- These bacteria multiply and invade the infection thread and finally reach up to the inner cortex where they enter into tetraploid cells and stimulate them to divide.
3. Formation of root nodule and bacteroids:
- The proliferating cells form a knob-like protuberance called root nodule. These bacteria multiply and colonize inside the tetraploid cells until the available cytosol is filled after which they become dormant.
- Each enlarged non-motile and dormant bacterium is called a bacteroid. A typical root nodule cell contains several thousand bacteroids.
4. Peribacteroid membrane and peribacteroid space:
- Usually the bacteroids occur inside the cytoplasm in groups. Each group is surrounded by a membrane called peribacteroid membrane. The space inside the cell surrounded by peribacteroid membrane is called peribacteroid space.
- A red pigment called Leghaemoglobin is filled outside the peribacteroid space in the cytosol of nodule cells.
- Leghaemoglobin is similar to haemoglobin in our RBCs, and has the ability to combine very rapidly with oxygen and thus acts as a very efficient O2 scavenger.
Also see Nitrogen Cycle
Mechanism of biological nitrogen fixation:
- There are some basic requirements of nitrogen fixation;
- Presence of enzyme nitrogenase and hydrogenase
- A protective mechanism for the enzyme nitrogenase against O2
- A non-heme iron protein, Ferredoxin as an electron carrier
- Hydrogen containing system like pyruvate, hydrogen, sucrose, glucose etc.
- A constant supply of ATP
- Presence of thiamine pyrophosphate (TPP), coenzyme-A, inorganic phosphate and Mg2+ as co-factors
- Presence of Cobalt and Molybdenum
- A carbon compound for trapping released ammonia
- In the process of biological nitrogen fixation by free living and symbiotic nitrogen fixers, the nitrogen molecule is progressively reduced step by step to ammonia by the addition of pairs of hydrogen atoms.
- The pyruvic acid serves as an electron donor (Hydrogen donor) in most of the cases. However, other electron donating systems such as hydrogen, sucrose, glucose etc. have also been shown to operate in different systems.
- In leguminous plants, glucose-6-phosphate molecule probably acts as a substrate for donating hydrogen.
- The overall process occurs in the presence of enzyme, nitrogenase, which is active in anaerobic condition.
- Nitrogenase consists of two sub-units;
- a non-heme iron protein commonly called Fe protein also called dintrogen reductase.
- and an iron-molybdenum protein called Mo-Fe protein or dinitrogenase
- Both the sub-units are needed for the activity of the enzyme. This Fe-protein component reacts with ATP and reduces Mo-Fe protein which then reduces nitrogen to ammonia.
- The oeverall biochemical reaction is as follows:
N2 + 8e– + 8 H+ + 16 ATP ———-> 2NH3 + H2 + 16 ADP + 16 Pi
- The product of nitrogen fixation is ammonia which is toxic to plants. The ammonium ions are however safely assimilated by higher plants.
- In most of the cases, the ammonium ions are transformed into amino acids and then translocated’
Ammonia + ∝-ketoglutarate +NADH ——-> Glutamate + NAD+ +H2O (Action of Dehydrogenase)
- In case of legumes, the ammonia is taken up by host and immediately assimilated into organic form (i.e. amino acids, amides or ureides) and then translocated through vascular tissues to other parts of the plant.
- Most of the ammonia produced from free living nitrogen fixers is either directly metabolized by the microorganisms, absorbed by the higher plants or converted to nitrates by the process of nitrification.
Nitrification:
- Ammonia is oxidized to nitrite and nitrate ions by a group of soil inhabiting nitrifying bacteria. e.g. Nitrosomonas and Nitrobacter.
- These nitrifying bacteria obtain their energy from the oxidation of ammonium and nitrite ions.
- The Nitrosomonas group oxidizes ammonia to nitrites;
NH4+ + 3/2 O2 <———–> NO2– + H2O + H+ -84Kcal ( By Nitrosomonas)
- The Nitrobacter group oxidizes nitrites to nitrates;
NO2– + 1/2 O2 ————> NO3– + -17.8 Kcal (By Nitrobacter)
- The nitrates (NO3–), produced by nitrification, are absorbed by higher plants and assimilated by the process called nitrate assimilation.
Nitrate assimilation in plants:
- The process of reduction of nitrate into ammonia is called nitrogen assimilation or nitrate assimilation.
- Absorption of nitrate by plant roots from the soil is a carrier-mediated, active and energy-dependent process.
- The nitrates absorbed by the plant roots get converted into amino acids and amides before being incorporating into proteins and other macromolecules.
- In most plants, the reduction of nitrate occurs in the root tissues and then transported to shoot through xylem whereas in others, the process occurs in leaves and stems. The overall summary equation of the reduction of nitrate to ammonia is as follows:
NO3– + 8e– + 10H+ ——–> NH4+ + 3H2O
- This reduction process consists of the following two distinct enzymatic steps:
- First step is the conversion of nitrate to nitrite. The reaction is catalyzed by the enzyme nitrate reductase (sulfhydryl containing molybdoflavohemoprotein). This step occurs in the cytosol outside any organelle and requires NADH (or in few species NADPH) as an electron donor, FAD as a prosthetic group, Cytochrome b557 as an electron carrier and Molybdenum (Mo) as an activator of enzyme. The pathway electron transfer in the reduction of nitrate to nitrite is as follows:
NADH + H+ + FAD ——–> NAD+ + FADH2
(or NADPH+ + H+) (or NADP+)
FADH2 + (Oxi) Mo ——> (Red) Mo + 2H+
(Red) Mo + 2H+ + NO3 ——–> (Oxi) Mo + NO2
-
- The second step is the reduction of nitrite to ammonium ion. The reaction is catalyzed by the enzyme nitrite reductase. The most probable electron donor in this reaction appears to be reduced Ferredoxin (an iron containing, non-heme protein of lower molecular weight). The Ferredoxin is reduced in the light reaction of photosynthesis in green leaves. The nature of electron donor in the non-green tissue (e.g. roots) is not known. In the non-green tissues some unknown electron carrier (probably FADH2 or NADPH + H+), generated in respiratory metabolism donates electrons. The overall reaction of nitrite reduction is as follows:
NO2 + 6e– + 8H+ ——–> NH4+ + 2H2O