- A compound in which a metal atom or ion is co-ordinated to two or more anions or neutral molecules is called co-ordination or complex compound.
- Complex compound retains its identity in its solid state as well as in the solution.
- In these compounds, the number of species surrounding the central metal atom is beyond its electrovalency or covalency. For example, in [Cu(NH3)4]SO4, the electrovalency of Copper is two, but it is surrounded by four NH3 molecules.
- A co-ordination compound generally contains one or more complex ions. For example;
[Cu(NH3)4]SO4 contains a complex ion, [Cu(NH3)4]2+
K4[Fe(CN)6] contains a complex ion, [Fe(CN)6]4+
[Ag(NH3)2]Cl contains a complex ion, [Ag(NH3)2]+
[Pt(NH3)4][PtCl4] contains two complex ions, [Pt(NH3)4]2+ and [PtCl4]2-
Complex ion:
- An electrically charged ion which consists of a central metal atom or ion surrounded by a group of ions or neutral molecules is called a complex ion. For example, [Ni(NH3)6]2+ is a complex ion in which the central Ni2+ ion is surrounded by six NH3 molecules.
- The complex ion carrying a positive charge is called cationic complex and the one with a negative charge is called anionic complex.
- The complex ion without any charge is called a neutral complex.
- Some more examples of complex ions are:
[Co(NH3)6]3+, [Cu(NH3)4]2+, [Ag(CN)2]–, [Fe(C2O4)3]3- etc.
Central atom or Central ion:
- The metal atom or ion to which two or more anions or neutral molecules are attached is called central atom or central ion.
- For example, in the complex [Co(NH3)6]3+, Co3+ is the central atom and in the co-ordination compound [Pt(NH3)4][PtCl4], Pt2+ is the central ion.
Ligands:
- The molecular or ionic species which gets attached directly to the central metal atom or ion during the formation of a complex is called a ligand.
- The ligands are attached to the central atom or ion through co-ordinate bonds. Therefore, ligands are also called the coordinating group, molecule or ion in a complex ion.
- The atom in the ligand which can donate the electron pair is called donor atom or coordinating atom. For example, in ammonia, nitrogen is the donor atom and in water, oxygen is the donor atom.
- The electron pairs donated by ligands are accommodated in vacant orbitals of the central metal atom to form a complex.
Types of ligands:
- A ligand may contain one or more donor sites. Depending upon the number of donor sites, ligands may be of the following types:
- Monodentate or unidentate ligands
- Bidentate ligands
- Polydentate ligands
1. Monodentate ligands:
- Ligands which donate only one pair of electrons and thus coordinate to the central atom or ion through only one atom are known as monodentate or unidentate ligands.
- Monodentate ligands have only one donor site. E.g. H2O, NH3, Br–, CN–, OH–, NO2–, CO etc.
2. Bidentate ligands:
- Ligands which have two donor atoms and therefore, have the tendency to attach to the central ion through two donor sites are called bidentate ligands.
- E.g. oxalate ion, Ethylenediamine, Dimethyl glyoximate ion
3. Polydentate ligands:
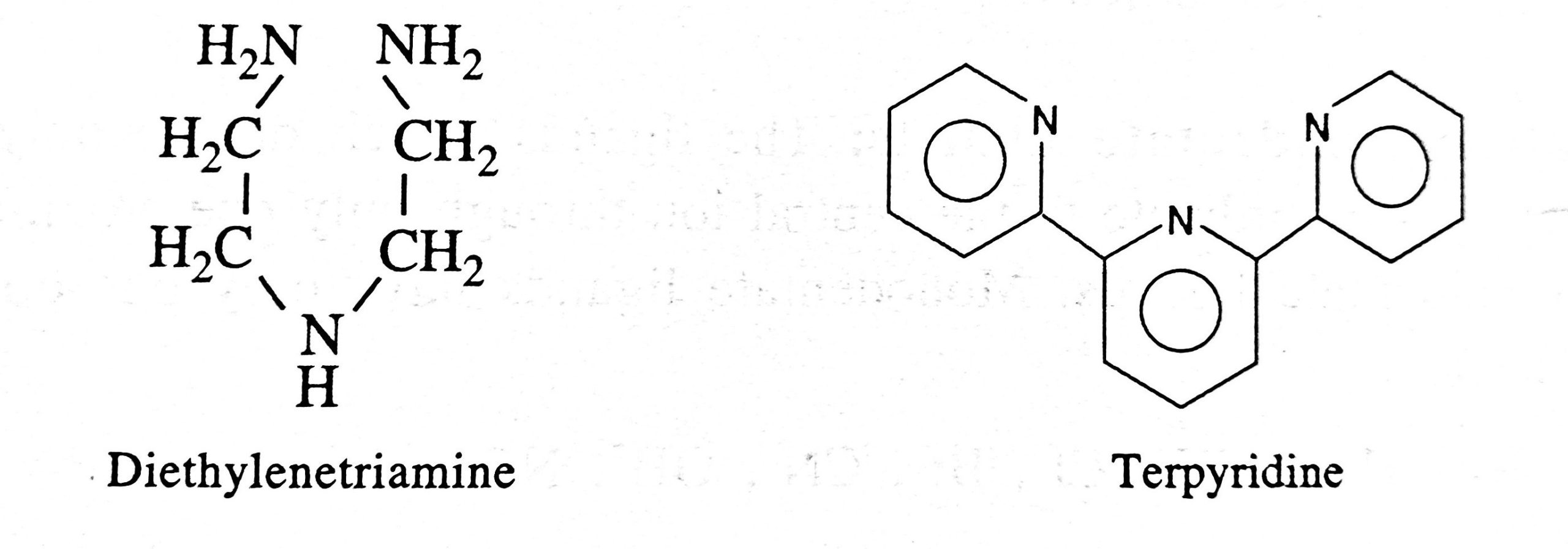
- Ligands which coordinate with the central ion through more than two donor atoms present in the molecule are called polydentate ligands.
- They may be tridentate, pentadentate and hexadentate having three, five and six donor sites respectively.
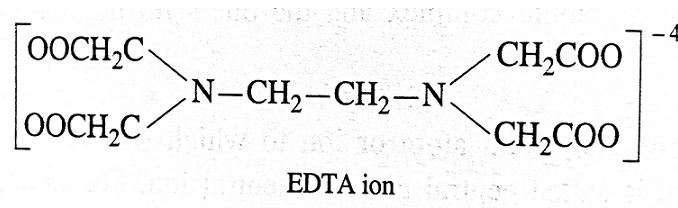
- For example, ethylenediamine tetraacetic acid (EDTA) is an important hexadentate ligand. EDTA ion binds through two nitrogen and four oxygen atoms of the four COOH groups to the central metal ion.
- Other important polydentate ligands are: Diethylenetriamine, Terpyridine