- Clinical research is a branch of healthcare science that performs clinical trials or carries out research experiments on people aiming at finding a solution to a disease.
- Clinical trials are research experiments that determine the safety and effectiveness or efficacy of medications, devices, diagnostic tests, treatments etc. and also monitor and evaluate their effects and outcomes on human health.
- Clinical research is different from clinical practice. In clinical practice, established treatments are used, while in clinical research evidence is collected to establish a treatment.
- Clinical trials determine the efficacy of:
- Drugs
- Vaccines and preventive treatment
- Biological products
- Surgical and radiation procedures
- Medical devices
- Gene therapy etc.
- Major interventions that require human research experimentation are drug testing and development of vaccine and its testing.
- The initial stages of clinical trial start in the laboratories with the production of drugs or vaccines and experimenting on animals and some human cell lines.
- When the first experiment is successful, the review team in the regulating body known as Food and Drug Administration (FDA) reviews and analyzes the data and decides whether to give approval for clinical research and testing directly on humans or not.
- According to the Food and Drug Administration (FDA), a complete clinical trial review team consists of the following professionals with their respective responsibilities:
Clinical trial Review Team:
- Project Manager: Coordinates the team’s activities throughout the review process, and is the primary contact for the sponsor.
- Medical Officer: Reviews all clinical study information and data before, during, and after the trial is complete.
- Microbiologist: Reviews the data submitted, if the product is an antimicrobial product, to assess response across different classes of microbes.
- Statistician: Interprets clinical trial designs and data, and works closely with the medical officer to evaluate protocols and safety and efficacy data.
- Pharmacologist: Reviews pre-clinical studies.
- Pharmacokineticist: Focuses on the absorption, distribution, and metabolism of drugs, and excretion processes. Interprets blood-level data at different time intervals from clinical trials, as a way to assess drug dosages and administration schedules.
- Chemist: Evaluates a drug’s chemical compounds. Analyzes how a drug was made and its stability, quality control, continuity, the presence of impurities, etc.
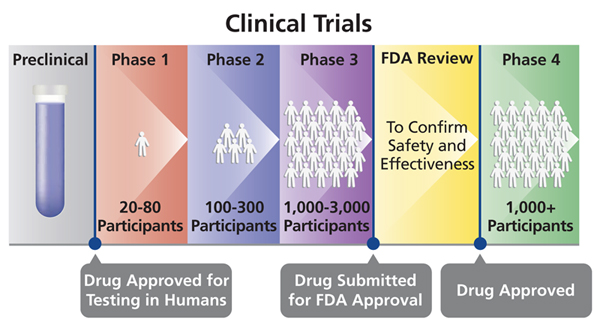
- The testing of the drug or vaccine or medical device can begin once the FDA approves human experimentation.
- These experiments are done in four to five phases where each phase is completely a separate trial although each phase builds on the previous phase.
- Each time a phase trial gets completed, the investigators must present their data for FDA approval before they continue to the next phase.
- The duration of clinical trial can vary based on the type of disease or infection.
- For example, cancer clinical trials are long and can be done in three phases according to the FDA, while those of novel emerging infections take about several months or at most a year (four to five phases).
Preparation for a clinical trial:
- Similar to a study plan, before any clinical trial, the investigators must prepare a research design, with specific research objectives. The steps that are involved in a clinical trial are known as protocols which have to be presented to the FDA for approval before beginning the clinical trials This protocols include:
- Choosing the selection criteria of participants who qualify for the study
- The number of participants to involve in the study
- Duration of the study
- Inclusion of a control group and how to limit research bias
- The dosage and duration of administrating the drug
- The assessment of the research trial should answer what? when? and what data is to be collected
- How to review and analyze the data
Participants in a clinical trial:
- To participate in a clinical trial, a participant must qualify for the study, meeting all the inclusion criteria of age, gender, the type and stage of the disease, previous treatment history, and any other medical conditions. The investigator must inform the participant on all possible guidelines (possible risks and outcomes) to participate in the study.
- People of all age groups including children can be involved in a clinical trial once their informed consent is taken.
- Participants can be healthy and sick individuals depending on the type of clinical interventions of investigation and the type of disease or infection. The exclusion and inclusion criteria are meant to identify appropriate participants, promote participants’ safety, and to ensure the researchers get the required information at the end of the study.
Different phases of Human clinical trials:
- Phase 0
- This is a clinical trial that is done on a very group of people (normally less than 15).
- The investigators will use a very small dosage of the medication ensuring that it is harmless before administering a higher dosage for the next phase.
- The outcomes of each individual from the experiment are closely monitored, reported and documented accordingly.
- If the individuals react to the medication differently, another preclinical research is done before making a decision on whether to continue or terminate the trial.
- In most trials, Phase 0 is not mandatory and hence investigators move directly to phase one (I) after preclinical research trials are approved.
- Phase I
- The investigators move to Phase I after a positive result outcome of phase 0.
- They use a larger group of people (20-80) with no underlying health conditions. This phase runs up to several months spending more time.
- In this phase, a larger dose of drug is used; basically the largest dose a human being can take without serious side effects.
- Close monitoring of the participants, and continuous reporting and documenting their body reactions is done in this phase.
- In this phase, investigators decide the route of the dug administration (intravenous, oral, or topical) based on which can be more effective or depending on the type of infection or disease of investigation.
- According to the FDA, if the medication is from trusted sources and approximately 70% successful, it can then move to the next phase, phase II.
- Phase II
- This phase studies test treatments that have been found to be safe in phase I but now need a larger group of human subjects to monitor for any adverse effects.
- It involves slightly more than 100 people, who have the condition of disease/infection under investigation.
- The dosage of treatment is the same as the one given at phase I. However, the population investigated for the trial is still not enough for demonstrating the overall safety of the medication but the data collected in this phase broadens the prospects of phase III.
- According to the FDA, about 30% of the drugs found to be safe in phase II move to Phase III.
- Phase III
- Studies are conducted on even larger populations and in different regions and countries and often this is the phase right before a new treatment is approved.
- It involves 300- 3000 participants with the condition the medication are meant to treat.
- This phase trials can last for a long period (as long as several years) because it involves a large group of participants in different regions.
- This phase trials aim at evaluating how the new medication works in comparison to other existing drugs treating the same condition, and therefore, the investigators must demonstrate the efficacy, safety, and effectiveness of the medication against that of the existing drugs, if any.
- Randomized studies are used whereby participants are randomly selected to form different sets or groups where one set receives the new medication while another set of participants called controlled placebo groups is administered with an identical but fake drug normally called sugar pills.
- Phase III trials are usually double-blind, which means that neither the participant nor the investigator knows which medication the participant is taking. This helps in eliminating bias during result interpretation.
- Rare and long-term side effects are more likely to show up during this phase because of larger number of participants and longer duration.
- If the investigators can be able to show that the medication is safe and effective, the FDA can approve it for use.
- At least 25-30% of drugs from trusted sources by the FDA move to Phase IV.
- Phase IV
- In this phase, studies and further testing of drugs in a wide population over a longer time frame is done, after the drug is approved by a country.
- Investigators use this phase to get more information about the medication’s long-term safety, effectiveness, and any other benefits.
Different Phases of Clinical Trials (Drugs testing and Development of vaccines) in Clinical Research
References:
- books.google.com>Clinical Research – Page 13
- https://www.fda.gov/patients/drug-development-process/step-3-clinical-research
- https://microbenotes.com/phases-of-clinical-trials-for-drugs-and-vaccine-development/