- A sugar consists of an anomeric carbon to which a functional group is attached.
- If the anomeric carbon consists of aldehyde as a functional group, the sugar is aldose and if ketone is attached, the sugar is ketose.
- Carbohydrates or sugars can be classified as either reducing or non-reducing on the basis of their reducing property.
- A sugar is said to be reducing if its anomeric carbon consists of a free aldehyde or ketone group.
- That’s why monosaccharides (with free aldehyde and ketone group in anomeric carbon) are called reducing sugars.
- In case of disaccharides, some are reducing while others are non-reducing. If anomeric carbon of both the monomers is involved in glycosidic bond, the disaccharide becomes non-reducing. E.g. sucrose and trehalose.
- If the anomeric carbon of any one of the monomers is free, the disaccharide becomes reducing. E.g. lactose and maltose.
- Almost all polysaccharides are non-reducing.
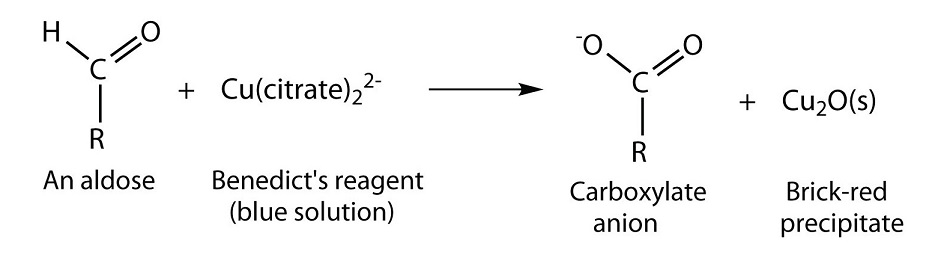
- Benedict’s test is one of the tests which is frequently employed to identify the reducing action of sugars.
- The reduction is much more efficient in alkaline medium than in the acidic medium.
Principle of Benedict’s test:
- Benedict’s test is performed by heating the reducing sugar solution with Benedict‘s reagent.
- The process of shifting of a hydrogen atom from one carbon atom to another in alkaline condition to produce enediols is known as tautomerization or enolization. The enediols are highly reactive, hence sugars in alkaline condition are powerful reducing agents.
- During the reaction, enediols reduce the cupric ions (Cu2+) of CuSO4 from Benedict’s reagent to cuprous ions (Cu+) which form a yellow precipitate of cuprous hydroxide or a red precipitate of cuprous oxide that separates from the solution.
- The intensity of red color and the volume of precipitate will be more if the concentration of reducing sugar is high in the solution.
Requirements:
- Sugar solutions (test solutions): 5 % Glucose, 5 % Sucrose
- Water (control)
- Benedict’s reagent:
-
- Benedict’s reagent is a deep blue alkaline solution of copper sulphate pentahydrate (CuSO4. 5H2O) in sodium carbonate (Na2CO3) and sodium citrate (Na3C6H5O7) and distilled water. Sodium carbonate renders alkaline conditions which are required for the redox reaction, while sodium citrate is a complexing agent which complexes with the copper (II) ions to avoid degradation into copper (I) ions during storage.
- Water bath
- Dry test tubes
- Pipettes
Procedure:
- Pipette out 2 ml (10 drops) of Benedict’s reagent into three clean and dry test tubes.
- Add approximately 1ml of each of the test solutions and water into each test tube having Benedict’s reagent.
- Place the test tubes over the boiling water bath for 3-5 minutes or heat the mixture directly over the flame.
- Observe the test tubes for color change in the solution or the formation of precipitate.
Observation and result interpretation:
- Positive Benedict’s test: color change from blue to brick red precipitate (glucose)
- Negative Benedict’s test: no change in color (sucrose) and water
- Since the intensity of red color and the volume of precipitate change with the concentration of reducing sugar in the solution, the result can be further interpreted as:
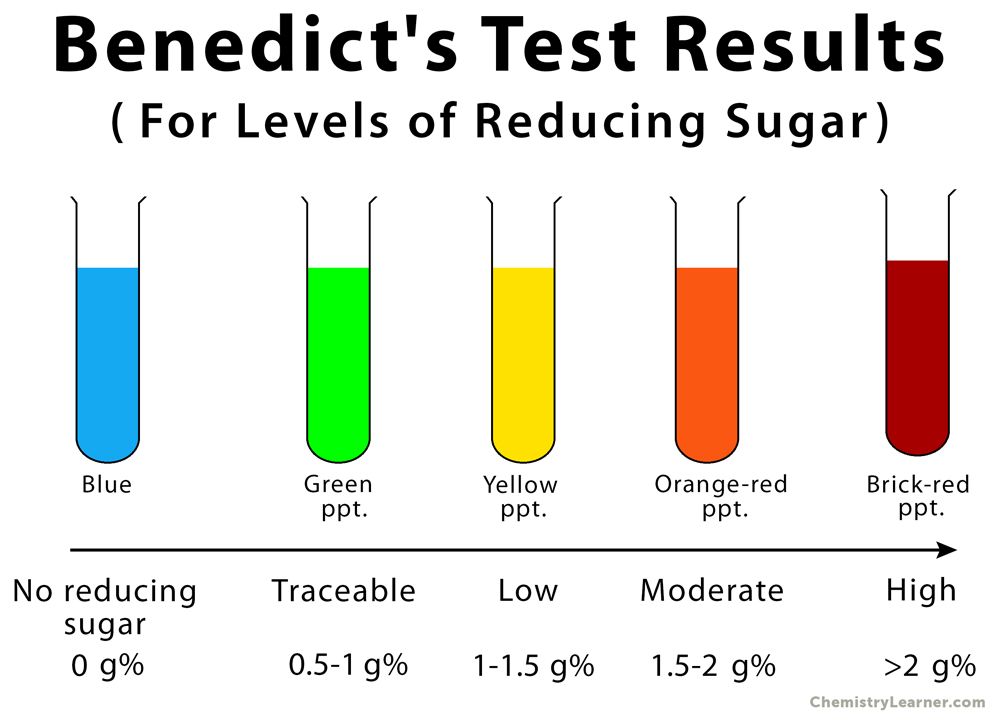
| Appearance of solution | The concentration of reducing sugar (g%) | Interpretation |
| Brick red with heavy precipitate | 2% or >2% | Large amount of reducing sugar is present |
| Brownish orange with red precipitate | 1.5% | Moderate amount of reducing sugar is present |
| Yellow with precipitate | 1% | Small amount of reducing sugar is present |
| Greenish blue and cloudy | 0.5% | Traceable amount of reducing sugar is present |
| Greenish blue with yellow precipitate | 0.25% | Traceable amount of reducing sugar is present |
| Blue color | 0% | Absence of reducing sugar |