- Acid-fast staining is one of the differential staining techniques commonly used for light microscopic examination of bacteria.
- It differentiates acid-fast bacteria from non-acid fast bacteria.
- It was developed by Ehrlich and later on modified by Ziehl and Neelsen, hence also known as Ziehl-Neelsen staining.
- It is specifically designed for a group of bacteria whose cell walls contain long chain fatty acids called Mycolic acids.
Principle:
- Mycobacteria contain large amount (60%) of Mycolic acid in their cell wall.
- Due to this high content of lipids, the cells become waxy and therefore most of the stains cannot penetrate into the cells of these bacteria by normal staining with aqueous based stains such as the Gram stain. i.e. Mycobacterium tuberculosis can’t be stained by using Gram staining technique.
- Mycolic acids render the cells resistant to decolorization, even with acid alcohol decolorizers. Thus, these bacteria are referred to as being acid-fast.
- Bacteria lacking cell walls fortified with mycolic accids cannot resist docolorization with acid alcohol and are categorized as being non-acid fast.
- Some degree of acid-fastness is shown by a few non-Mycobacterial bacteria, such as Nocardia, and coccidian parasites, such as Cryptosporidium spp.
- Acid-fast staining uses the following reagents in sequential order.
- Primary Stain (0.3% Carbol fuchsin):
- Carbol fuchsin, a dark red stain in 5% phenol is soluble in the mycolic acid of mycobacterial cell wall.
- It can penetrate through to the cytoplasm which is enhanced by the application of heat (in Ziehl-Neelsen method).
- The Kinyoun method, a modification of the Ziehl-Neelsen method, uses a wetting agent (Tergitol) or a higher concentration of phenol to this stain. It reduces the surface tension between the cell wall of mycobacteria and the stain and hence enhances intracellular penetration of carbol fuchsin.
- The Kinyoun method doesn’t require heating hence also called a “cold method”.
- After primary staining, all cells will appear red.
- Decolorization Solution (Acid-Alcohol; 3% HCl + 95% Ethanol):
- Acid-fast bacteria will be resistant to decolorization when mycolic acid solidifies, hence prior to decolorization, the smear is cooled .
- Carbol fuchsin is more soluble in mycolic acid than in the decolorizing agent. So, after decolorization, acid fast bacteria will stay red while non-acid fast bacteria become decolorized (colorless).
- Counterstain (0.3% methylene blue or malachite green):
- Methylene blur or malachite green is used as a final reagent to stain previously decolorized cells.
- As only non–acid-fast cells undergo decolorization, they may now absorb the counterstain and take on its blue or green color, while acid-fast cells retain the red color of carbol fuchsin.
Requirements:
- Fresh culture sample of Mycobacterium smegmatis and Staphylococcus epidermidis
- Primary stain: carbol fuchsin
- Decolorizing agent: acid alcohol (3% HCl +95% ethanol)
- Counter stain: methylene blue/ malachite green
- Bunsen burner
- Inoculating loop
- Microscope
- Distilled water
- Soft cotton or tissue paper
- Grease free glass slides
Procedure (Ziehl-Neelsen technique):
- Fix smears on heated surface (600C) for at least 10 minutes.
- Flood smears with carbol fuchsin (primary stain) and eat to almost boiling by performing the procedure on an electrically heated platform or by passing the flame of a Bunsen burner underneath the slides on a metal rack. The stain on the slides should steam.
- Allow the slides to sit for 5 minutes after heating. Do not allow them to dry out. Wash the slides in distilled water (note: tap water may contain acid-fast bacilli). Drain off excess liquid.
- Flood slides with decolorizer (3% HCl +95% ethanol) for approximately 1 minute. Check to see that no more red color runs off the surface when the slide is tipped.
- Add a bit more decolorizer for very thick smears or those that continue to release red dye.
- Wash thoroughly with water and remove the excess.
- Flood slides with malachite green or methylene blue (counter stain) and allow to remain on surface of slides for 1 minute.
- Wash with distilled water and stand slides upright on paper towels or tissue paper to air dry. Don’t blot dry.
- Examine microscopically using 100X oil immersion objective.
Microscopic examination:
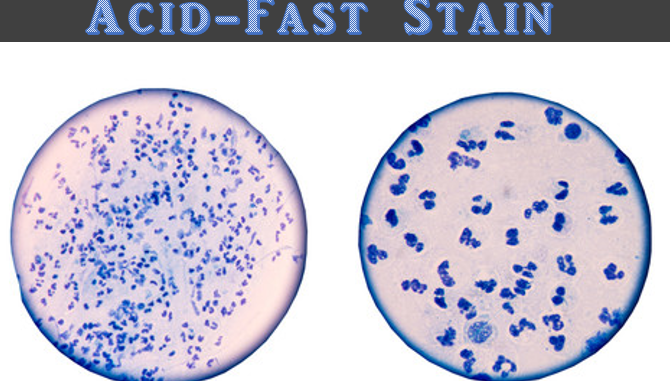
- Acid-fast: Red, straight or slightly curved rods, occurring singly or in small groups e.g. Mycobacterium smegmatis
- Non-acid fast: Blue color; In addition, background material should stain blue e.g. Staphylococcus epidermidis